This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
IOLs + SI Injectors
AM60
Optimized Asphericity Single-Piece IOL
The patented design of the iVis IntraOcular Lenses exploits core aspheric design principles of CIPTA® custom refractive surgery.
The AM60’s aspheric optics is designed to enhance visual function while minimizing the positional sensitivity common to other aspheric lenses.
Key Features
- Core aspheric CIPTA® principles applied to IOLs
- Patented aspheric optical design, surgically optimized
- Anti-PCO Square-Edge
- Surgeon-friendly, small incision, one-step injection

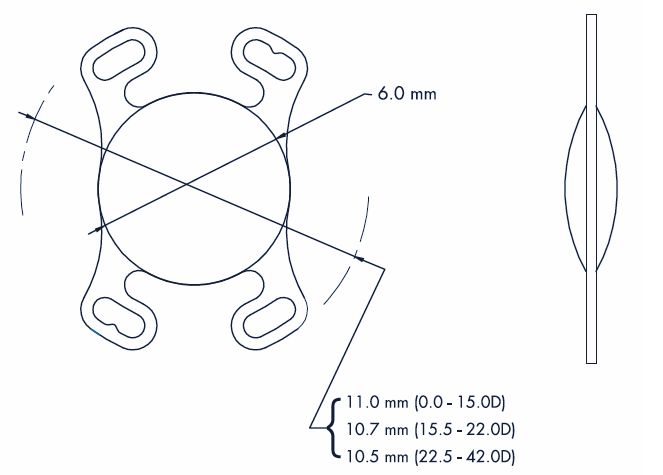
General Specifications
– A Constant: 118°
– A.C.D.: 4.96 mm
– Incision Size : 1.8 mm
– Diopter range: From 0.0 D to +42.0 D
– Diopter details:
From 0.0 D to +10.0 D x 1.0 D increments
From +10.0 D to +30.0 D x 0.5 D increments
From +30.0 D to +42.0 D x 1.0 D increments
Haptic Specifications
– Length:
11.0 mm (from + 0.0 D to +15.0 D)
10.7 mm (from +15.5 D to +22.0 D)
10.5 mm (from +22.5 D to +42.0 D)
– Angle: 0°
Optic Specifications
– Geometry: Equal Biconvex Aspheric
– Optic edge: Square
– Diameter: 6.0 mm
– Anterior profile: Optimized Asphericity
– Volume : Constant across power range
Additional Specifications
– Type: Aspheric Single Piece
– Material: Hydrophilic Acrylic
– Filter: UV blocker
– Implant: Posterior Chamber
– Insertion: Injector or Manual
– Refractive index: 1,457 @ 35°C (Fully Hydrated)
– Light transmission: >95%
Optical hydrophilic acrylic with UV blocker (26% water content)
Manufacturing Specifications
– Sterilization: Steam
– Sterilization validity: 36 months
– Packaging: Single piece in glass vial
– Storage: Room temperature
AA60
Optimized Asphericity Single-Piece IOL
The patented design of the iVis IntraOcular Lenses exploits core aspheric design principles of CIPTA® custom refractive surgery.
The AA60 is designed to allow an extremely wide range of refractive powers from -10D up to +42D.
Key Features
- Core aspheric CIPTA® principles applied to IOLs
- Patented aspheric optical design, surgically optimized
- Anti-PCO Square-Edge
- Surgeon-friendly, small incision, one-step injection
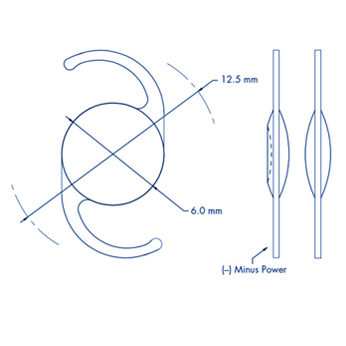
General Specifications
– A Constant: 118°
– A.C.D.: 5.10 mm
– Incision Size : 2.4 mm
– Diopter range: From -10.0 D to +42.0 D
– Diopter details:
From -10.0 D to +10.0 D x 1.0 D increments
From +10.0 D to +30.0 D x 0.5 D increments
From +30.0 D to +42.0 D x 1.0 D increments
Haptic Specifications
– Length: 12.5 mm
– Angle: 0°
Optic Specifications
– Geometry: Equal Biconvex Aspheric
– Optic edge: Square
– Diameter: 6.0 mm
– Anterior profile: Optimized Asphericity
– Volume : Constant across power range
Additional Specifications
– Type: Aspheric Single Piece
– Material: Hydrophilic Acrylic
– Filter: UV blocker
– Implant: Posterior Chamber
– Insertion: Injector or Manual
– Refractive index: 1,457 @ 35°C (Fully Hydrated)
– Light transmission: >95%
Optical hydrophilic acrylic with UV blocker (26% water content)
Manufacturing Specifications
– Sterilization: Steam
– Sterilization validity: 36 months
– Packaging: Single piece in glass vial
– Storage: Room temperature
B60
Asphericity Single-Piece IOL
The B60’s aspheric optic is designed to improve visual outcomes with respect to a standard spherical IOL.
Features
- Surgeon-friendly, one-step injection implantation
- Symmetrical bi-convex aspheric optic design
General Specifications
– A Constant: 118°
– A.C.D.: 5.10 mm
– Incision Size : 2.4 mm
– Diopter range: From 0.0 D to +42.0 D
– Diopter details:
From 0.0 D to +10.0 D x 1.0 D increments
From +10.0 D to +30.0 D x 0.5 D increments
From +30.0 D to +42.0 D x 1.0 D increments
Haptic Specifications
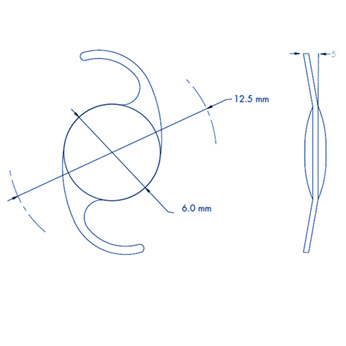
– Length: 12.5 mm
– Angle: 5°
Optic Specifications
– Geometry: Equal Biconvex Aspheric
– Optic edge: Square
– Diameter: 6.0 mm
Additional Specifications
– Type: Aspheric Single Piece
– Material: Hydrophilic Acrylic
– Filter: UV blocker
– Implant: Posterior Chamber
– Insertion: Injector or Manual
– Refractive index: 1,457 @ 35°C (Fully Hydrated)
– Light transmission: >95%
Optical hydrophilic acrylic with UV blocker (26% water content)
Manufacturing Specifications
– Sterilization: Steam
– Sterilization validity: 36 months
– Packaging: Single piece in glass vial
– Storage: Room temperature


